


Next: The Schrödinger Equation
Up: The Motivation for Quantum
Previous: Quantization of Electronic Angular
Einstein had shown that the momentum of a photon is
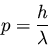 |
(4) |
This can be easily shown as follows. Assuming 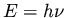 for a photon
and
for a photon
and
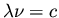 for an electromagnetic wave, we obtain
for an electromagnetic wave, we obtain
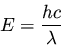 |
(5) |
Now we use Einstein's relativity result E = m c2 to find
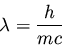 |
(6) |
which is equivalent to equation (4). Note that mrefers to the relativistic mass, not the rest mass, since the rest
mass of a photon is zero. Since light can behave both as a wave (it
can be diffracted, and it has a wavelength), and as a particle (it
contains packets of energy 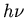 ), de Broglie reasoned in 1924 that
matter also can exhibit this wave-particle duality. He further
reasoned that matter would obey the same equation (4)
as light. In 1927, Davisson and Germer observed diffraction patterns
by bombarding metals with electrons, confirming de Broglie's
proposition.
), de Broglie reasoned in 1924 that
matter also can exhibit this wave-particle duality. He further
reasoned that matter would obey the same equation (4)
as light. In 1927, Davisson and Germer observed diffraction patterns
by bombarding metals with electrons, confirming de Broglie's
proposition.
de Broglie's equation offers a justification for Bohr's assumption
(2). If we think of an electron as a wave, then for
the electron orbit to be stable the wave must complete an integral
number of wavelengths during its orbit. Otherwise, it would interfere
destructively with itself. This condition may be written as
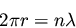 |
(7) |
If we use the de Broglie relation (4), this can be
rewritten as
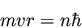 |
(8) |
which is identical to Bohr's equation (2).
Although de Broglie's equation justifies Bohr's quantization
assupmtion, it also demonstrates a deficiency of Bohr's model.
Heisenberg showed that the wave-particle duality leads to the famous
uncertainty principle
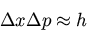 |
(9) |
One result of the uncertainty principle is that if the orbital radius
of an electron in an atom r is known exactly, then the angular
momentum must be completely unknown. The problem with Bohr's model is
that it specifies r exactly and it also specifies that the orbital
angular momentum must be an integral multiple of 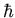 .
Thus the
stage was set for a new quantum theory which was consistent with the
uncertainty principle.
.
Thus the
stage was set for a new quantum theory which was consistent with the
uncertainty principle.



Next: The Schrödinger Equation
Up: The Motivation for Quantum
Previous: Quantization of Electronic Angular